Uncategorized
“All I want for Christmas…”
 |
| Jagatman’s amputation |
It’s a leg.
With all the shopping we do at Christmas, imagine wishing only for a leg. When I visited Nepal in September, I reported on one of our “boys,” Jagatman. He’s only 26, same age as my daughter Tara. Unlike her, his life is so uncertain and frail. He has hemophilia; he lives in a country that purchases no factor.
 |
| Jagatman at work |
And he lost his leg due to an untreated bleed. Through Project SHARE, we provided factor for the surgery to remove his leg, to save his life. And he’s so grateful to us. Sigh, the fates must be angry. The April 25 earthquake ripped apart his brick home. I stood before the rubble of what once was the home of the Rajbchak family.
 But he has a mobile phone repair shop (Save One Life helped get him started with funding) and I saw him eagerly at work. He’s good! Industrious, focused and determined to succeed. It must have taken a lot of courage to ask me to help him purchase a leg. He’s outgrown his current prosthetic; it hurts him. Given the high tolerance for pain these boys have, it must re
But he has a mobile phone repair shop (Save One Life helped get him started with funding) and I saw him eagerly at work. He’s good! Industrious, focused and determined to succeed. It must have taken a lot of courage to ask me to help him purchase a leg. He’s outgrown his current prosthetic; it hurts him. Given the high tolerance for pain these boys have, it must re
ally hurt him. He earns only about $500 a month from the shop, and must pay rent. The leg he showed me in a colorful brochure costs almost $4,000. A fortune. A king’s ransom.
 |
| With brother Monsoon, who also has hemophilia |
But I promised this handsome young man that by Christmas, we’d buy him that leg. What better gift?
And last week we made good. The money was wired and he will soon have his leg. Despite a chronic disorder, limited access to medicine and poverty, Jagatman is beating the odds. His attitude is astounding; his determination like Rocky’s. His focus like a laser beam.
And he’ll never know that the gift he’s given me is so much more than anything I can ever give him.
Merry Christmas to our friends in Nepal, and those with bleeding disorders everywhere!
Great Book I Just Read
Best rock bio I have ever read. Told by the iconic guitarist of the Rolling Stones, Richards almost seems as surprised by his life’s journey as you are bound to be after reading this. Meticoulously detailed, Richards shares his humble beginnings, his teen encounter with life long rock partner Mick Jagger, their rocky road to fame, the drugs, the alcohol… but what makes this bio so different is first, the Stones are one of the greatest rock bands in the world, from the early 60s and still playing! Second, Richards delves into the music: his love of American blues, what he wrote, why, how he got ideas, the chords, the notes, the guitars. This is what I have been wanting to know: how do these musicians create? He’s a voracious reader, loves his library, and lives in Connecticut, a few hours from me. He’s a survivor for sure, and what a story he has to tell. Five/five stars!
It’s Just a Phase: Clinical Trials of New Products
Deep Down Dark: The Untold Stories of 33 Men Buried in a Chilean Mine, and the Miracle That Set Them Free [Kindle]
Hector Tobar
Pulse on the Road in Baton Rouge
 |
| Zoraida and Kelly before the presentation |
 |
| Laurie Kelly and attendee Shawn Whelan |
 |
| Speakers Michelle Rice (NHF), Kelly Lynn Gonzales and Laurie Kelley |
Said Carl, an attendee with hemophilia, “I’ve heard and read lots of testimonials from people and their problems with insurance, doctors and hospitals. But these ladies really opened my heart on how some people are really neglected.”
 |
| Laurie and Jan Hamilton, founder of HFA |
| We end the year with a pep talk! Read your insurance policy annually, document your insurance inquiries and treatments, watch for hidden costs, and put aside an emergency fund for unexpected escalating costs and to help pay for hospitalizations. Be vigilant! |
 |
| Voodoo Shop! |
 |
| Louisiana Riverboat |
 |
||
Andrew Jackson Statue
|
Blood: Time to Donate!
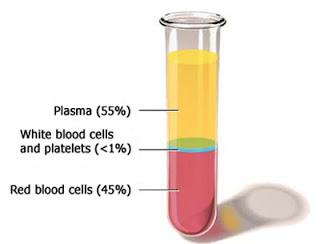 National Hemophilia Foundation recommends recombinant factor as the standard of choice for treatment of hemophilia, but did you know that many factor products used to treat
National Hemophilia Foundation recommends recombinant factor as the standard of choice for treatment of hemophilia, but did you know that many factor products used to treathemophilia are developed from human blood, specifically human plasma? In fact, one person with hemophilia can require up to 1,200 plasma donations for a one year’s supply of factor
products. Plasma products are especially important for those undergoing immune tolerance therapy to treat inhibitors, for those with von Willebrand disease and for those in developing countries.
approximately 55 percent of total blood volume. A single liter of plasma yields
coagulation factors essential for blood clotting, immunoglobulins used to
combat viruses and bacterial infections, and albumin, a major plasma protein
that regulates blood volume and other essential functions.
are used in everyday medicines, emergency and critical care situations, as well
as preventive medicine. Albumin, for example, is used to treat burns, shock,
trauma, liver conditions and cardiopulmonary illnesses; immunoglobulins are
indicated for Rh incompatibility, pediatric HIV, hepatitis, and animal bites.
of plasma-derived product production. This is due primarily to its biologic
nature: plasma protein therapies are not interchangeable, have no generic
variations or substitutions, and are defined as sole-source biologic products
by global regulators.
oversight measures are in place—including collection, processing, and storage
and handling requirements—to ensure plasma donor health, as well as product
purity and efficacy for patients.
in the U.S. Collectively, the Food and Drug Administration (FDA) and its Center
for Biologics and Research (CEBR) are responsible for regulatory oversight of
the U.S. blood supply. Blood collection centers are either registered or
licensed by the FDA, and are held to quality standards comparable to those of pharmaceutical
manufacturers. Blood establishments located outside of the U.S. that import or
offer for import blood products are also required to register with the FDA.
used for transfusions, as well as for the manufacture of pharmaceuticals
derived from blood and blood components. CEBR develops and enforces quality
standards, inspects blood establishments and monitors reports of errors,
accidents and adverse clinical events.
followed by purification and virus inactivation. Fractionation is a time
consuming and complex process that extracts, or “fractions off,” specific
plasma proteins that have a proven health benefit. Fractionation requires
multiple processing steps, which involve manipulating solution pH, temperature,
ionic strength and alcohol concentration.
virus inactivation, a complex purification processes that includes prion
removal, nanofiltration, solvent/detergent
treatments and incubation, to ensure sterility and purity of the final product.
The complete manufacturing process, from plasma collection at a donor
center to the FDA’s lot release, takes seven to 12 months.









