Factor your way: empowering you to achieve your goals
Sponsored Content
This is a paid public announcement from Takeda and does not constitute an endorsement of products or services. When you click on the links in this blog entry, you will be directed to the bleedingdisorders.com website. LA Kelley Communications always advises you to be a savvy consumer when contacting any company; do not reveal identifying information against your will.
Submitted by Takeda
What is hemophilia?
 Hemophilia is a rare genetic bleeding disorder that prevents blood from clotting normally.1
Hemophilia is a rare genetic bleeding disorder that prevents blood from clotting normally.1
At the time of a bleed, proteins in your blood called clotting factors form a clot and stop the bleeding.2,3 However, if you have hemophilia, your blood lacks clotting factors (such as factors VIII and IX), and as a result, you may bleed for a longer time after an injury than you would otherwise.4,5
Replacing what’s missing
The current standard of care for hemophilia is factor replacement therapy.6 Factor therapy is a proven treatment with decades of real-world use and an established safety record.7,8
Factor therapy replaces the missing blood-clotting proteins that are naturally found in your blood.9 It is administered via an intravenous injection (directly into your bloodstream), making these proteins available immediately for use.
Factor therapy can be used in different situations such as on-demand (to treat an ongoing bleed), prophylaxis (to prevent bleeds before they occur), and before or after surgery.6,10
Other treatments for hemophilia
The first non-factor therapy option more recently became available.11 To reduce bleeding, it mimics part of the function of missing clotting proteins by bridging other factors. It is administered subcutaneously and is intended for prophylactic use.
Individualizing your treatment with factor
With factor treatment, your healthcare provider (HCP) can individualize your 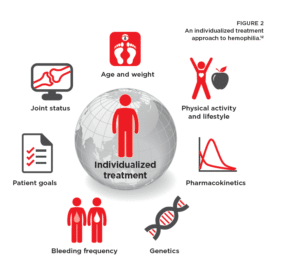 regimen to align with your unique needs and help you achieve treatment goals.
regimen to align with your unique needs and help you achieve treatment goals.
When determining an appropriate treatment plan, your HCP looks at how factor is processed in your body; this is called pharmacokinetics (PK).13 Many factors such as body weight, age, joint status, activity levels, and bleeding frequency are used to better understand how much and how often you require factor.12
A treatment tailored to your lifestyle and activities may help make it easier for you to stick to your prophylaxis. This may help preserve your joint health by significantly reducing bleeding.12
CASE STUDY
Individualizing factor therapy can make a world of difference
Josh
Age 15 years
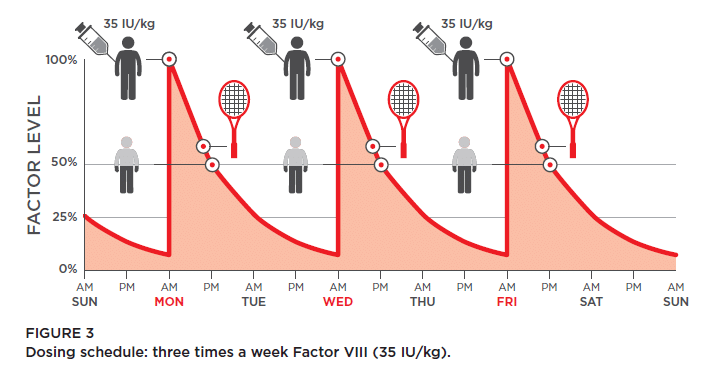
Josh is an active teen who enjoys playing sports. He is currently taking factor every other day (25 IU/kg) but has recently been experiencing more bleeds, particularly in his ankles. At his HCP visit, Josh mentions that he has increased his tennis practice from once weekly to 3 nights/week.
Due to an increase in Josh’s activity level, and a considerable growth spurt, he may not always be receiving optimal coverage from his current dosing regimen. His HCP recommended adjusting Josh’s dose of factor VIII and changing his infusion schedule.
After taking a few blood samples, they were able to determine what Josh’s factor levels would be at various time points following an infusion. They agreed to an individualized dosing schedule that was appropriate for Josh’s current activity level. Josh’s new dose is 35 IU/kg three times a week.
Since switching to the new dosing schedule, Josh’s bleeding has been reduced even with his increased activity.
Visit bleedingdisorders.com for more information about hemophilia and individualized factor therapy.
Supporting literature:
- Livnat T, Barg AA, Levy-Mendelovich S, Kenet G. Rare bleeding disorders—old diseases in the era of novel options for therapy. Blood Cells Mol Dis. 2017;67:63-68.
- Smith SA, Travers RJ, Morrissey JH. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50(4):326-336.
- Berg JM, Tymoczko JL, Stryer L. Many enzymes are activated by specific proteolytic cleavage. Biochemistry. 5th ed. New York, NY: WH Freeman; 2002. https://www.ncbi.nlm.nih.gov/books/NBK22589/. Accessed April 18, 2019.
- Hemophilia. National Heart, Lung, and Blood Institute (NHLBI). https://www.nhlbi.nih.gov/health-topics/hemophilia. Accessed April 17, 2019.
- Hemophilia. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/hemophilia/symptoms-causes/syc-20373327. Accessed April 17, 2019.
- Rodriguez-Merchan EC. What’s new in orthopedic surgery for people with hemophilia. Arch Bone Jt Surg. 2018;6(3):157-160.
- Pipe SW. New therapies for hemophilia. Hematology Am Soc Hematol Educ Program. 2016;(1):650-656.
- Franchini M. Current management of hemophilia B: recommendations, complications and emerging issues. Expert Rev Hematol. 2014;7(5):573-581.
- Rolstad EB. Perceptions of men with moderate to severe hemophilia regarding the management of their chronic disorder and utilization of community-based support. Am J Mens Health. 2015;9(6):486-495.
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187-197.
- National Hemophilia Foundation. MASAC document #255. Available at: www.hemophilia.org. Accessed May 2, 2019.
- Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia. 2014;20(5):607-615.
- Pharmacokinetics. The Free Dictionary by Farlex. http://medicaldictionary.thefreedictionary.com/pharmacokinetics. Accessed April 17, 2019.
Copyright © 2019 Takeda Pharmaceutical Company Limited. All rights reserved. All trademarks are the property of their respective owners.
S48266 05/19
Patient Newsletter Article 20190226 0.6


 In the years that followed, we advanced the treatment of hemophilia A, hemophilia B, hemophilia A or B with inhibitors, von Willebrand disease, and acquired hemophilia A with significant developments. Many of these were firsts: the first recombinant factor VIII treatment, the first needleless transfer device, the first recombinant factor VIII treatment free of blood-based additives, the first recombinant treatment for people with von Willebrand disease, and the first recombinant porcine factor VIII for acquired hemophilia.6-9
In the years that followed, we advanced the treatment of hemophilia A, hemophilia B, hemophilia A or B with inhibitors, von Willebrand disease, and acquired hemophilia A with significant developments. Many of these were firsts: the first recombinant factor VIII treatment, the first needleless transfer device, the first recombinant factor VIII treatment free of blood-based additives, the first recombinant treatment for people with von Willebrand disease, and the first recombinant porcine factor VIII for acquired hemophilia.6-9 Thanks to the many contributions that have been made in the past, and which Shire continues to make, to the treatment of bleeding disorders, Shire’s vision for patients with a bleeding disorder is closer to realization than ever before.
Thanks to the many contributions that have been made in the past, and which Shire continues to make, to the treatment of bleeding disorders, Shire’s vision for patients with a bleeding disorder is closer to realization than ever before. And the innovation continues. Research and development is going strong with 20 ongoing clinical trials in bleeding disorders, including one in gene therapy, as well as advancements in other novel therapies. Shire has engaged hundreds of the world’s leading scientists, researchers, and patient support specialists to help them.5
And the innovation continues. Research and development is going strong with 20 ongoing clinical trials in bleeding disorders, including one in gene therapy, as well as advancements in other novel therapies. Shire has engaged hundreds of the world’s leading scientists, researchers, and patient support specialists to help them.5 Most fundamentally, Shire is collaborating with the bleeding disorders community, including patient associations that have enabled the diagnosis of more than 30,000 hemophilia patients around the world.5 Shire has listened to, learned from, and championed their needs. This bleeding disorders community is our community. It’s why Shire is always pushing ahead, proactively shaping the future of bleeding disorders and continually elevating care for patients.
Most fundamentally, Shire is collaborating with the bleeding disorders community, including patient associations that have enabled the diagnosis of more than 30,000 hemophilia patients around the world.5 Shire has listened to, learned from, and championed their needs. This bleeding disorders community is our community. It’s why Shire is always pushing ahead, proactively shaping the future of bleeding disorders and continually elevating care for patients.

